Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only when the liquid surface is in contact with gas (such as the air). If the surface is between two liquids (such as water and oil), it is called "interface tension."
The surface can hold up a weight, and the surface of a water droplet holds the droplet together, in a ball shape. Some small things can float on a surface because of surface tension, even though they normally could not float. Some insects (e.g. water striders) can run on the surface of water because of this. This property is caused by the molecules in the liquid being attracted to each other (cohesion), and is responsible for many of the behaviours of liquids.
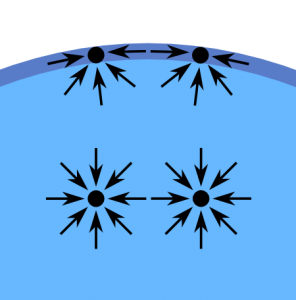
The cohesive forces among the liquid molecules cause surface tension. In the bulk of the liquid, each molecule is pulled equally in every direction by neighbouring liquid molecules, resulting in a net force of zero. The molecules at the surface do not have other molecules on all sides of them and therefore are pulled inwards. This creates some internal pressure and forces liquid surfaces to contract to the minimal area.
Examples of Surface Tension
- Drops of water. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. The shape of the drops is caused by the surface tension of the water. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. In the absence of gravity, the drop would minimize the surface area in order to minimize tension, which would result in a perfectly spherical shape.
- Insects walking on water. Several insects are able to walk on water, such as the water strider. Their legs are formed to distribute their weight, causing the surface of the liquid to become depressed, minimizing the potential energy to create a balance of forces so that the strider can move across the surface of the water without breaking through the surface. This is similar in concept to wearing snowshoes to walk across deep snowdrifts without your feet sinking.
- Needle (or paper clip) floating on water. Even though the density of these objects is greater than water, the surface tension along the depression is enough to counteract the force of gravity pulling down on the metal object. Click on the picture to the right, then click "Next," to view a force diagram of this situation or try out the Floating Needle trick for yourself.



